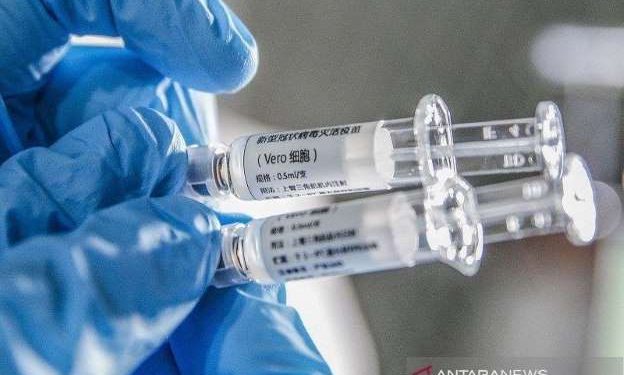
Sinovac Biotech Chief Executive Yin Weidong said nearly 90 percent of its employees and their families had received the company’s experimental Covid-19 vaccine, which was developed under China’s emergency program.
Emergency use programs are aimed at specific groups, including medical personnel and workers in the food market and in the transport and service sectors.
Sinovac, which is an artificial Corona vaccine is currently in Phase III trials.
Yin Weidong offers the vaccine candidate to an estimated 2,000 to 3,000 employees and their families.
Where employees and their families can also be considered as volunteers.
The data gathered from these programs can provide evidence that the vaccine is safe.
However, such data are not part of the clinical trial protocol and will not be used as the main ingredient, which is being reviewed by the authorities in vaccine approval.
Side effects after being injected with the CoronaVac vaccine include fatigue, fever, and pain, mostly with mild symptoms, based on the results of an intermediate trial involving 600 participants and published last month.
Of the number of Covid-19 vaccine candidates being developed by various countries, not one has passed the final stage to date.
Large-scale trials must be conducted to prove the effectiveness and safety of the vaccine before it is widely used in the community.